We induced a deep state of anesthesia in rats by means of a ketamine/medetomidine peritoneal injection, and analyzed the time course of the correlation between the brain activity in different areas while anesthesia spontaneously decreased over time.
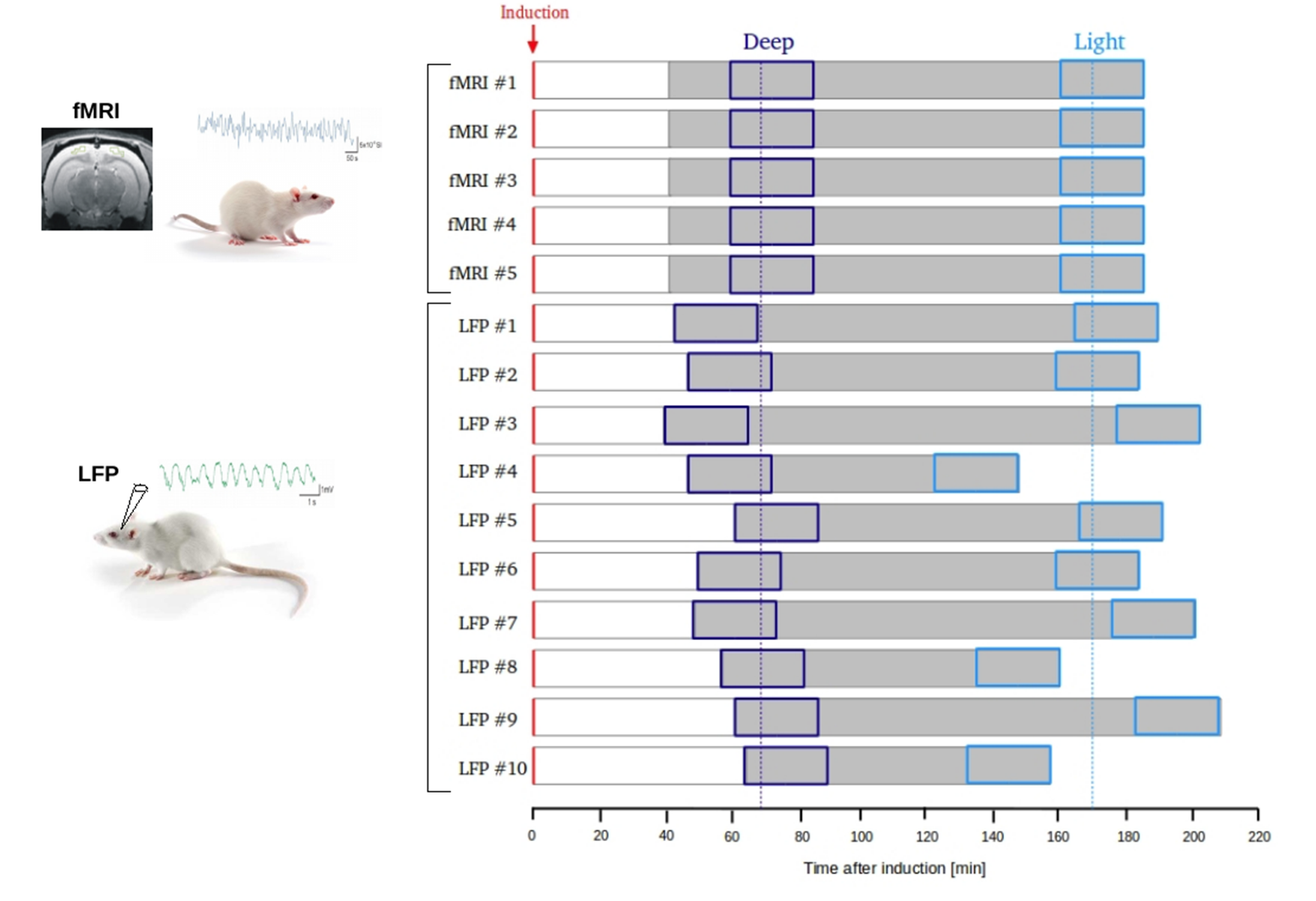
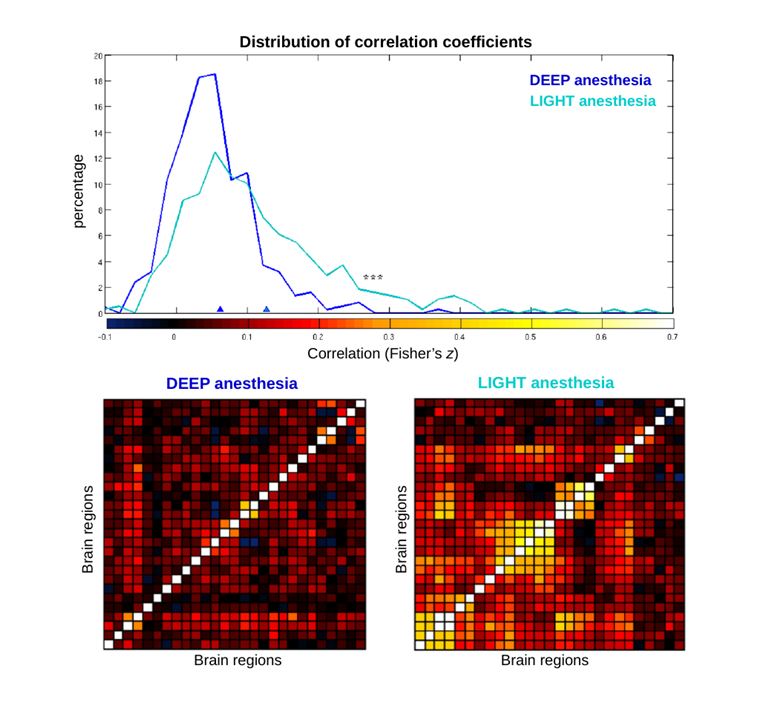
We then compared results separately obtained from fMRI and local field potentials (LFP) under the same anesthesia protocol, finding that while most profound phases of anesthesia can be described by overall sparse and mainly local correlations, during lighter states different frequency-specific functional networks emerge, endowing the gradual restoration of structured large-scale activity seen during rest.
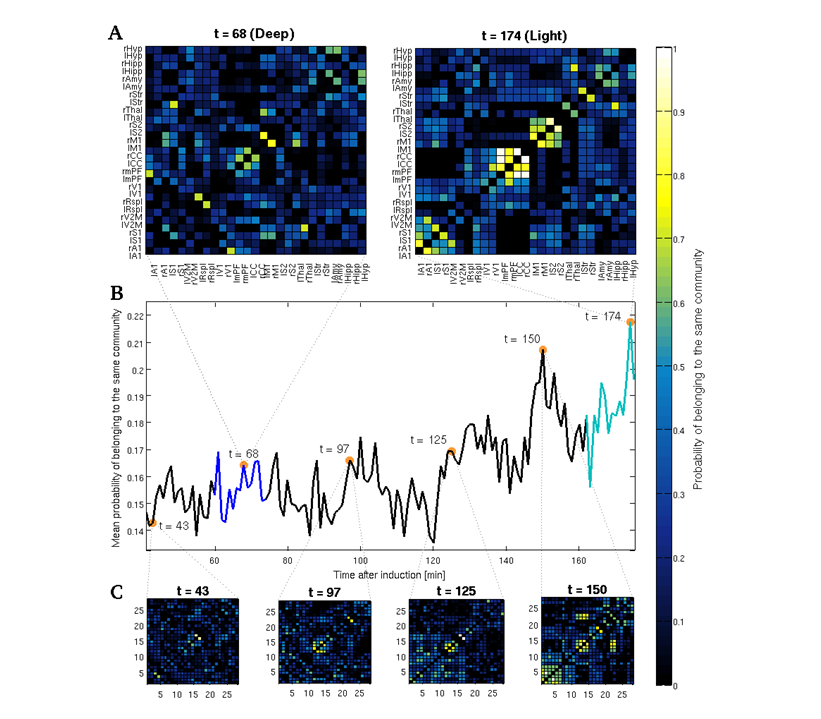
In order to capture the overall changes in communities' structure taking place during the gradual fading of anesthesia from the individual to the group level, we first iterated 10,000 times the community detection algorithm on the full (28 x 28) FC matrix obtained from each sliding window of each animal, and then computed the a posteriori probability that each pair of ROIs had of belonging to the same community across all iterations and all animals in each sliding window. We thus calculated, for each one of the obtained probability matrices, the mean a posteriori probability of belonging to the same community, in order to obtain a rather simple summary of the overall changes in the structural stability of different communities over the course of anesthesia.
Noteworthy, the LFP results show that those areas belonging to the same functional network (which interestingly appears to be the rat’s Default-Mode Network) exhibited sustained correlated oscillations around 10 Hz throughout the protocol, suggesting the presence of a specific functional backbone that is preserved even during deeper phases of anesthesia. In order to evaluate the frequency-specific coupling between areas belonging to the same resting-state network (mPF-CC) and regions that do not (A1-S2, referred to as “uncoupled”) in deep and light anesthesia, we computed the correlation between the envelopes of homologous band-limited signals obtained from two different regions, to which we refer to as band-limited correlations (BLCs). BLCs can be seen as an extension of the classical Functional Connectivity (Friston et al. 1993) in the frequency domain and can be used to quantify the degree of co-variation between neuronal oscillations of two distant cortical regions at a given frequency (Brookes et al. 2011, Hipp et al. 2012, Cabral et al. 2014b).
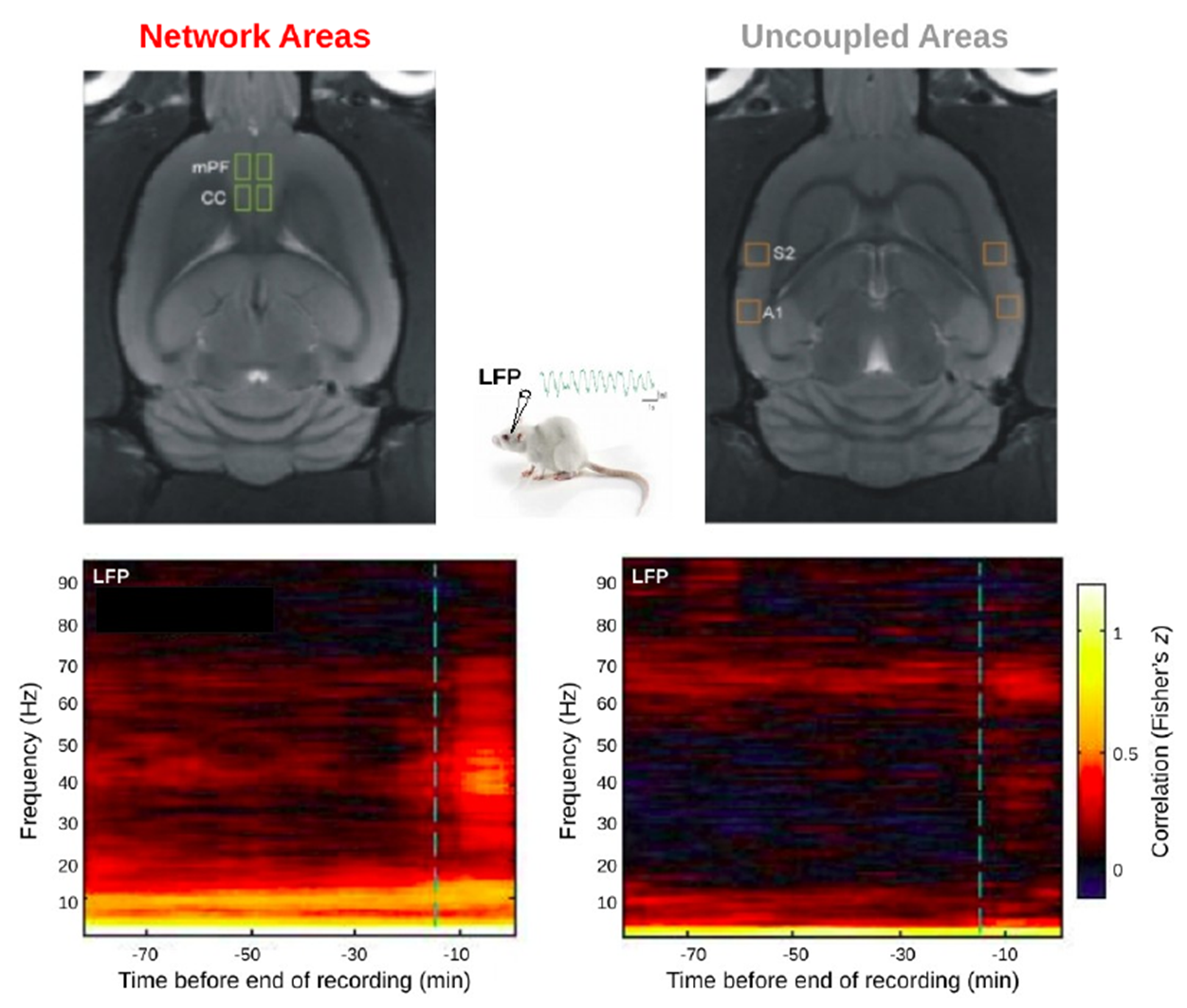
The progressive emergence from deep anesthesia is characterized by a progressive increase in correlated large-scale low-frequency fluctuations, as well as by an enhancement in the local coupling of band-limited oscillations between areas participating to the same functional network. On the other hand, more profound phases of anesthesia are marked by a decrease in differentiated activity. Progressive fading of anesthesia is mirrored by the gradual flourishing of highly organized spontaneous brain activity, being the default-mode network one of the first networks to emerge. Nonetheless, we observed that local frequency-specific connectivity between areas participating to the same functional networks is preserved also during deeper phases of anesthesia, indicating a partial maintenance of brain functional organization even during states of deep sedation.
